Rituximab is typically administered with methotrexate (MTX) for the treatment of rheumatoid arthritis (RA). About one-third of people who receive infusions of rituximab + MTX have an infusion reaction after the first exposure.1-3 As many as 41% develop an infection after an infusion of rituximab, but serious infections are rare.1,3,4 Other very rare, but serious, side effects include serious skin reactions and a neurological complication called progressive multifocal leukoencephalopathy.1,5
Infusion Reactions
Manufacturer’s package inserts for rituximab report that 32% of rituximab (+MTX) treated patients (n=540) had an infusion reaction within 24 hours of first exposure compared to 23% of placebo (+MTX) (n=398) in pooled placebo-controlled studies.1 After the second infusion, the rates drop to 11% and 13%, respectively. The pattern of symptoms includes headache, chills and fever, skin rash, dyspnea, pruritus, hypotension, nausea, weakness, rhinitis, and angioedema of the tongue and throat. Bronchospasm can also occur, but this is less common. Dosage adjustments (stopping or slowing of infusions) were required in 10% of people receiving rituximab and 2% receiving placebo after their first course. Less than 1% of both groups had a severe acute infusion-related reaction. Severe reactions may include hypotension, angioedema, hypoxia, bronchospasm, acute respiratory distress syndrome, heart attack, ventricular fibrillation, cardiogenic shock, anaphylaxis, or death.
Edwards et al. conducted a double-blind randomized controlled study of 161 patients with RA who received either oral MTX, rituximab infusions, infusions of rituximab plus cyclophosphamide, or rituximab plus methotrexate.2 Each group received oral placebos and/or infusion placebos to blind them to the study group to which they were assigned. In each group, 30% to 45% of individuals had reactions after the first infusion. The lowest event rate (30%) occurred in patients taking MTX monotherapy and the highest rate (45%) was in patients taking rituximab monotherapy. The reaction rate for people taking rituximab alone or in combination was 36% overall, but 85% to 90% of these reactions were characterized as mild or moderate.
Infections
According to the package insert, in placebo-controlled studies, 37 of 540 (7%) patients on rituximab + MTX reported upper respiratory infections, compared to 23 of 398 (6%) on placebo + MTX.1 Overall, 39% of patients in the rituximab+ MTX group experienced an infection of any type compared to 34% of patients in the placebo +MTX group. Common infections were nasopharyngitis, urinary tract infections, bronchitis, and sinusitis. The rate of serious infections was 2% in the rituximab + MTX-treated patients and 1% in the placebo + MTX group. Pneumonia, cellulitis and urinary tract infections were the most common serious infections (≥0.5%). Fatal infections included pneumonia, sepsis, and colitis.
The Randomized Evaluation of Long-Term Efficacy of Rituximab in RA Trial was a randomized placebo-controlled study of treatment with rituximab.6 All patients were treated with background MTX, and 311 patients on rituximab were compared to 209 on placebo after 24 weeks. The rate of serious infections at 24 weeks after a single infusion of rituximab + MTX resulted in a serious infection rate of 5.2 per 100 patient-years compared to 3.7 per 100 patient-years in the placebo group.
Van Vollenhoven et al. pooled data from a global clinical trial program of patients with active RA.4 As of December 2012, 3,595 patients had received a mean of four courses of rituximab over 11 years. (14,816 patient-years). They calculated a pooled overall serious infection rate of 3.76/100 patient-years. The most frequent serious infection reported was pneumonia.
Other Common Side Effects
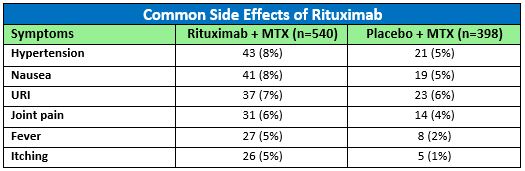
In addition to infusion reactions and infections, side effects that were pooled from Phase II and Phase III study populations of rituximab (+MTX) in RA are summarized in the table.1 The most common adverse reactions occurring in 5% or more of patients treated with rituximab (+MTX) were hypertension, nausea, upper respiratory tract infection (URI), joint pain, fever, and itching. The number of rituximab treated RA patients experiencing these reactions compared to those taking a placebo in clinical studies up to week 24 (pooled) are included in Table 1.
Rare Serious Side Effects
Severe, sometimes life-threatening skin reactions have occurred after infusion with rituximab including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), but this is extremely rare.1 Chen et al. conducted a literature search of papers published between 1950 and 2017 to identify case reports of severe cutaneous reactions among patients taking anticancer drugs and immunotherapies.7 Two case reports of SJS, two cases of SJS-TEN, and one case of TEN were found to have occurred among patients taking rituximab in this search.
Case reports of progressive multifocal leukoencephalopathy (PML) occurring in patients with RA taking rituximab have prompted experts to examine the relationship between this diagnosis and the presence of inflammatory disease and rituximab therapy.5 Because of the very small numbers involved, at present, the best estimate of the risk of PML is approximately one in 25,000 people exposed to rituximab.
Rare, but sometimes fatal, cardiac arrhythmias have been reported in patients taking rituximab.1 According to the package insert, in the pooled placebo-controlled studies, the proportion of patients with serious cardiovascular reactions was 1.7% and 1.3% in the rituximab and placebo treatment groups, respectively. People with RA are already at increased risk of cardiovascular events, so monitoring throughout infusion is recommended and rituximab should be stopped if an event occurs.
Rituximab has been shown to reactivate hepatitis B infection in patients with a past history of this infection.8 A retrospective study was conducted among RA patients in Taiwan, where the incidence of hepatitis B infection is more common. Of 44 RA patients treated with rituximab, 44 had evidence of previously resolved hepatitis B infection, and four (9.1%) of these infections were reactivated after the first cycle of rituximab. Reactivation occurred after a mean of 25 months after the first infusion.
References
- Rituxan- rituximab injection, solution [package insert]. San Francisco, CA: Genentech, Inc.; 2020.
- Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350 (25): 2572-2581.
- Smolen JS, Keystone EC, Emery P, et al. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 2007; 66 (2): 143-150.
- van Vollenhoven RF, Emery P, Bingham CO, 3rd, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol 2010; 37 (3): 558-567.
- Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 2011; 68 (9): 1156-1164.
- Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006; 54 (9): 2793-2806.
- Chen CB, Wu MY, Ng CY, et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res 2018; 10: 1259-1273.
- Tien YC, Yen HH, Chiu YM. Incidence and clinical characteristics of hepatitis B virus reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for rheumatoid arthritis. Clin Exp Rheumatol 2017; 35 (5): 831-836.



.png)
